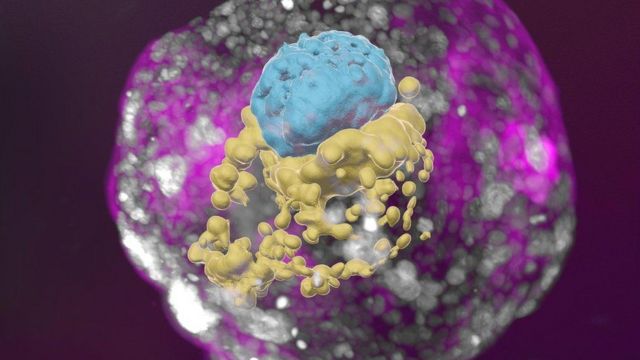
This is a model of a human embryo grown from stem cells: the embryonic cells are blue, the yolk sac cells are yellow, and the placental cells are pink.
Israeli scientists have succeeded in creating a miniature living organism that is virtually indistinguishable from a human embryo in its early stages of development. However, unlike the actual embryo, which is formed by the fusion of a sperm and an egg and attaches to the uterine wall, the creation of his “lab copy” did not require any of the above.
According to scientists at the Weizmann Institute of Israel, the “embryo model” they have grown from stem cells looks absolutely textbook, exactly like a healthy human embryo two weeks after conception. As it grew, it even produced hormones that scientists use in a pregnancy test to determine pregnancy. A pregnancy test performed in the laboratory showed a positive result.
Scientists are creating embryo models to study the first days after conception of new human life in the most ethical way. In the first few weeks after the sperm fertilizes the egg, the most dramatic events take place that will have an enormous impact on the future of the human being. It is during this time that a cluster of virtually identical cells, with no specific function, transforms into what can be recognized as a baby on subsequent ultrasound scans. However, during this crucial period most miscarriages occur and congenital defects of the future organism are laid down, and it is very poorly understood.
“It’s a black box, and it’s not a cliché – our knowledge is very limited,” says Professor Jacob Hanna of the Weizmann Institute in Israel. “We explain quickly, simply, and clearly what happened, why it matters, and what will happen next.” The number of offers should remain: episodes. End of story. Podcast Advertising.
The path of human embryo research is fraught with legal, ethical, and technical pitfalls. As a result, a rapidly developing field of science has emerged that simulates the natural development of human embryos. The results of this research have been published in the journal Nature.
The authors say they have created the first “complete” model of an embryo that mimics all the major structures that appear in the early stages of development. “This is really a classic image of a 14-day-old human embryo,” says Professor Hanna. According to him, no one has ever done anything like this before.
Instead of sperm and an egg, the starting material was so-called naive stem cells, which were reprogrammed with the help of a series of chemical substances to transform into four types of cells that are formed at the earliest stage of human embryo development. In total, the scientists mixed 120 such cells in the required ratio, then left them alone and began observing.
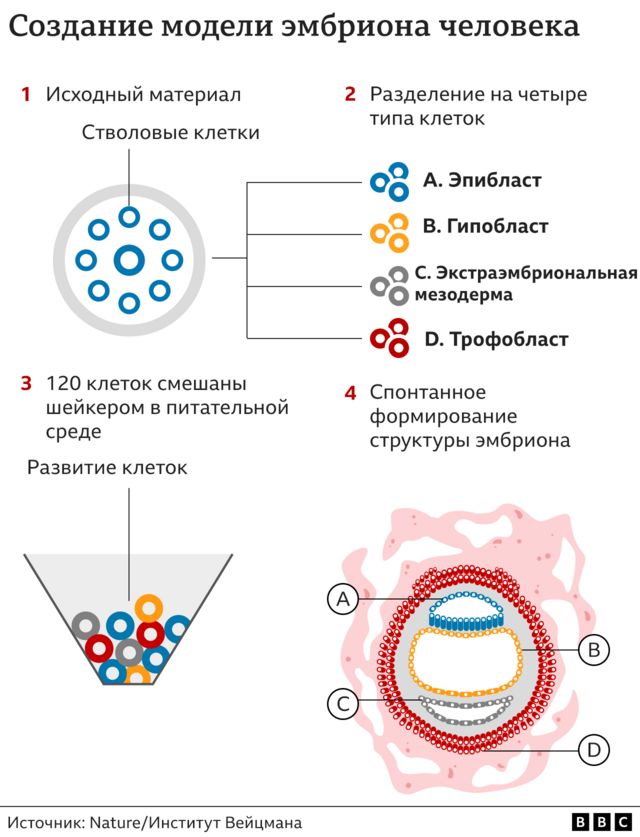

Approximately 1% of the steel mixture spontaneously assembled into a structure similar, though not identical, to a human embryo. “I give credit to the cells: all you have to do is make the right mixture and put them in the right environment, and everything will work out for them,” says Professor Hanna. “This is an amazing phenomenon. The embryo models were allowed to grow and develop until they were comparable to a typical human embryo on the 14th day after fertilization. In many countries, this is the legal limit after which normal human embryos cannot be used for research. (After the 14th day, the process of gastrulation begins, when the characteristics of the future organism are actually established. From this point on, for example, the formation of twins becomes impossible).
Despite my late video call, Professor Hanna gives me a tour with undisguised passion in her voice, highlighting the three-dimensional “exquisite architecture” model of the embryo. I see the trophoblast, the upper layer of the blastocyst, which would become part of the placenta and nourish the fetus during normal development. It has cavities called lacunae through which the mother’s blood carries nutrients to the baby. There is also a yolk sac, which performs some of the functions of the liver and kidneys, and a two-layered embryonic disk – one of the key features of this stage of embryonic development.
It is hoped that embryo models will help scientists understand how different types of cells develop, observe the earliest stages of organ development firsthand, and explain the origins of hereditary and genetic diseases. For example, this first study has already shown that other parts of the embryo simply do not form if they are not surrounded by placental cells. There is a view that these models will help improve the results of in vitro fertilization (IVF) if doctors can understand why some embryos develop and others do not. They may also be useful for testing the effects of different drugs on pregnancy to determine which are safe.
Professor Robin Lovell-Badge, who studies embryonic development at the Francis Crick Institute in the UK, told me that these embryo models “look quite good” and “quite normal. “I think it’s good, I think it’s done very well, it all makes sense, and I’m very impressed with the work,” he remarked. However, according to Professor Badge, it is necessary to ensure that more than 1% of the cells are collected in the model. The 99% failure rate definitely needs improvement. With such a high failure rate, it’s difficult to explain problems related to miscarriage and infertility when the model just doesn’t “come together” on its own in most cases.
The work also raises the question of whether it is possible to simulate the development of an embryo after the 14-day stage. This would not be illegal, even in the UK, because the models of embryos are not legally considered to be actual embryos. “Some people welcome it, some people don’t like it,” says Professor Badj.
Professor Alfonso Martinez Arias of the Faculty of Experimental and Medical Sciences at Pompeu Fabra University in Barcelona noted that this is an “incredibly important study. “This experiment made it possible for the first time to construct a complete structure of a human embryo from stem cells” in the laboratory, “thus opening the door to studying the processes that lead to the formation of a human embryo, the body plan,” he said.
Researchers emphasize that it would be unethical, illegal, and physically impossible to achieve a viable pregnancy using these embryo models – these 120 cells simply cannot develop to the point where they could be successfully implanted in the uterine lining.