This week the Medical Council refused to prescribe the drug “Zolgensma” to Mark Ugrehelidze from Krasnodar, which costs 121 million rubles. The child’s family had been collecting money for his treatment for a year and a half. According to the boy’s father, the doctors refused to prescribe “Zolgensma” because Mark is already being treated with another drug. The family intends to appeal to the Prosecutor’s Office and the Investigative Committee in order to obtain the desired treatment.
Mark Ugrehelidze (who will be four years old on February 9) has moderate grade 2 spinal muscular atrophy. Since the spring of 2020, the boy has been treated with the drug “Risdiplam” – initially provided free of charge by the manufacturer Roche, and then purchased by the state charitable fund “Krug Dobra” (Circle of Goodness). In addition to the use of “Risdiplam”, Mark’s parents launched a fundraising campaign for the purchase of “Zolgensma” – another drug to alleviate the course of the disease. For more than a year, the necessary amount of 121 million rubles was not collected. The fundraising was completed with the help of the fund of the former mayor of Ekaterinburg Yevgeny Roizman – supported by journalist Yulia Latynina, writer Boris Akunin, football commentator Vasily Utkin and others. The foundation of the fundraising was made up of private transfers from ordinary people. On January 31, Evgeny Roizman announced that the required amount had been raised. But raising enough money for the medication proved insufficient.
Spinal muscular atrophy (SMA) is a rare genetic disease that causes muscle wasting in humans. It is caused by a mutation in the SMN1 gene, which is responsible for the growth and survival of motor neurons. In patients with SMA, these neurons die every day – people gradually lose their motor activity, eventually leading to paralysis and suffocation. There are currently only three drugs that can slow or stop the progression of the disease: “Spinraza”, “Risdiplam” and “Zolgensma”. All of them are registered in Russia (the last one was registered on December 9, 2021). Before registration, only the “Circle of Goodness” fund had the right to purchase the drugs.
“Zolgensma” is manufactured by Novartis and is considered the most expensive drug in the world. It is administered only once, unlike other drugs that must be taken for life. The principle of action of “Zolgensma” is based on the fact that the drug essentially replaces the non-functioning SMN1 gene with its copy in the body, which normalizes the production of the protein responsible for the survival of neurons. The drug took 23 years to develop. The manufacturers justify its high price by the expensive development process and the fact that the drug “radically changes the lives of patients suffering from this devastating disease and their families.

Another drug for SMA is being developed by the Russian biotechnology company BIOCAD. According to the manufacturer, only the first stage of development has been completed, and the company is preparing for preclinical trials of the drug ANB-4, which has a similar mechanism of action to “Zolgensma”. As the developers assure, its price will be lower than that of foreign analogues. The president of the “Circle of Good” Alexander Tkachenko called on Russian manufacturers to speed up their work, and the Internet speculated that the refusals of medical commissions regarding “Zolgensma” were connected with the future release of a Russian-made drug. Currently, the Circle of Good Foundation, initiated by Vladimir Putin, provides medicines for children with spinal muscular atrophy who are under 18 years of age.
Please note that the number of offers remains the same. As of the beginning of December 2021, more than a thousand children with SMA, including 21 children with Zolgensma, have received treatment funded by the Foundation. We explain quickly, simply, and clearly what happened, why it matters, and what will happen next. the episodes End of story: Podcast Advertising.
According to Malkhaz Ugrekhelidze, Mark’s father, it is impossible to independently prescribe treatment with “Zolgensma” – only on the recommendation of a medical commission. Moreover, the manufacturers can sell and distribute the drug in Russia only through five licensed medical centers “with a guarantee that these centers are ready to administer the drug”. “Zolgensma” is not aspirin, you can’t just go to the pharmacy and buy it. It requires a doctor’s recommendation,” Mark’s father told the BBC.
“Before the registration of “Zolgensma” in Russia in December 2021, it could be used only on the basis of a decision of a federal council. After the registration, a decision of a regional council became sufficient. However, the regions still do not have the courage to [prescribe the drug] on their own and ask federal experts. They rejected us on February 3. As the man says, the consultation was held in a telemedicine format. The reason for the refusal, according to him, was that the child was already receiving “Risdiplam” and there was a “positive dynamic”. “So they think it is not advisable to prescribe another drug,” Ugrekhelidze complains. “And when you start asking for a full justification, the doctors don’t give it, they just rely on their opinion – ‘inadvisable’, that’s it.”
Mark’s parents have not yet received a final document after the February meeting, but they already have a “similar” document. This is not the first time Mark has refused treatment for Zolgensma. The previous council took place in early November 2021 – at that time, private funds for the purchase of the drug had not yet been collected, and the Ugrekhelidze family relied on funding from the Circle of Good Foundation. The refusal came for similar reasons (BBC has a copy of the conclusion): the child is noted to have a “positive effect” from taking “Risdiplam”, manifested as an “increase” in motor activity. At the same time, the commission drew attention to “violations” – “motor underdevelopment”, scoliosis of the spine, valgus deformity of the feet. According to doctors, it is “impossible” to eliminate bone deformities by gene replacement therapy (i.e. administration of “Zolgensma”). This conclusion upsets Malkhaz Ugrehelidze. He considers such information about his child’s health as “false” – the man claims that the day before the medical commission his son did not undergo any tests, and the preparation for it included only a “visual examination” by the attending neurologist in Krasnodar. “What other irreversible changes? Our doctor told me she did not provide that information. Where did they get that from?” – he asks. Malkhaz Ugrehelidze was also upset that an independent neurologist, the head of the “Helping Children with SMA” fund, Alexander Kurmyshkin, was not allowed to attend the February council meeting, which Mark’s family insisted on. This specialist has been observing the child for a year and a half and actively advises families with children with SMA all over the country. But Kurmyshkin managed to break through for the telemedicine session, albeit briefly. “He went in and asked the experts, ‘Do you think that Zolgensma will be unsafe for the child’s health?’ – recalls the boy’s father. And they answered him that no, they do not think so. We asked to have this noted in the record, but we were refused. And then the screens were turned off. On the air of “Echo of Moscow”, Alexander Kurmyshkin suggested that the refusal to appoint the drug could be associated with “money movement”. “Orphan diseases are very expensive, and unfortunately it is not the government that controls this, but certain commercial groups that have powerful lobbies in the government,” he said. “So if we raise money ourselves and manage it ourselves, and decide which drugs to prescribe based on the interests of the child, rather than a commercial company with which there is an agreement, it drastically changes their plans. We meet the interests of the patients and do not come to them to negotiate how much percentage we should give them in advance”.
Mark’s father, Ugrehelidze, believes that “Zolgensmu” will not be prescribed for the boy in order to avoid setting a precedent. If a positive decision is made, he says, the family will have the opportunity to demand free procurement through “Krug Dobra” (Circle of Goodness). And that could set off a chain reaction. “And then the fund will have to explain to children all over the country why they were not prescribed “Zolgensma” when it was approved for us,” says Ugrehelidze. “Not many people would want to spend hundreds of millions of rubles on a mass purchase of the drug.”
As the BBC found out, the refusal to buy “Zolgensma” came not only to Mark’s family. There are other children who have collected funds for an expensive injection, but it is not prescribed to them. Alexander Kurmyshkin says that there are “about a dozen” of them. One-and-a-half-year-old Volodya Sologub is one of these children. According to his mother Zoe, the prescription for “Zolgensma” was rejected three times, the last time also in early February this year. However, the family had collected funds for the commercial purchase of the drug in early January 2022.
“At first we applied for the purchase of “Zolgensma” through the “Krug Dobra” organization,” recalls Zoya Sologub. “We were turned down, but we tried again because we didn’t want to be accused of not trying to get the drug for free while the fundraising was going on. In addition, the criteria for participating in the Foundation’s purchasing were changing. But I still believed we would be denied. The Circle of Good Foundation did not immediately decide who should buy “Zolgensma” for the treatment of spinal muscular atrophy (SMA). In the first months of the Foundation’s work in 2021, there were very strict selection criteria. In particular, for the Foundation to pay for the medication, the age of the patient with SMA of the first degree of severity (the most destructive and dangerous) should not exceed six months. For SMA of the second degree, it was 12 months, and there were no age restrictions for the third degree, but this type of disease is usually found in adult patients who are not prescribed “Zolgensma”.
On the “Circle of Good” account on Instagram, many parents complain that they cannot receive “Zolgensma” according to the foundation’s rules. On December 9, 2021 the Fund canceled the age criterion. Now the restriction for the use of “Zolgensma” can be the weight of the child exceeding 21 kg, the child being on ventilation for more than 16 hours a day for more than 14 days, feeding through a gastrostomy or nasogastric tube, as well as the detection of antibodies to adenovirus in the patient, which is contained in “Zolgensma”. According to the recommendations of the “Circle of Kindness”, the patient can receive “other methods of pathogenetic treatment”, but only before “Zolgensma” is administered to him. After that, the child’s representatives should refuse further treatment with other drugs. In other words, it is allowed to use “Zolgensma” after conditional “Risdiplam”, but not vice versa. At the same time, according to the instructions approved by the Ministry of Health, no studies on interaction with other drugs have been conducted, and the experience of using the drug in combination with other drugs for SMA treatment is limited. The instructions also indicate the need to carefully monitor liver function – the main side effects of this medication are associated with impaired liver function.
Zoya Sologub hopes for “Zolgensma” also because, according to her observations (every few months Volodya receives “Spinraza” – BBC note), the current treatment leads to “regressions” in the child’s development. About a month before the next dose is administered, her son “weakens”, it becomes harder for him to eat and breathe, says the woman. “This happened after the fifth dose,” Zoya recalls. “The child became so weak that he couldn’t eat properly. He choked on the food, it got into his lungs, and he developed aspiration pneumonia. We spent a week in the intensive care unit, and the doctors gave us various indications of the possible outcome. The pneumonia occurred in September 2021 – after that, the child never returned to a normal diet. He still receives it through a feeding tube.
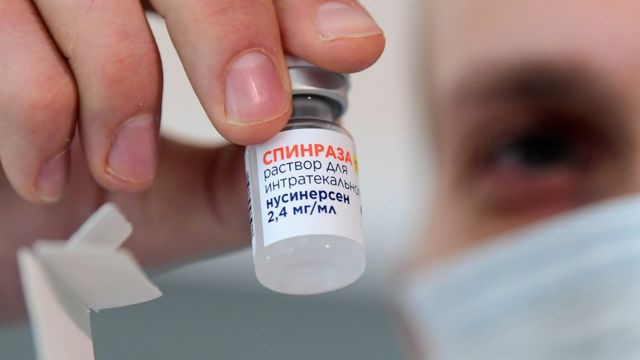
“The swallowing function is preserved, but it is weakened,” says Zoya Sologub. “If my son had been given Zolgensma in the tenth month, when we asked for it, everything would have been fine. But we didn’t meet the criteria. And now it’s like a closed loop: if the child doesn’t respond well to Spinraza, then Zolgensma won’t help. And if the child does well on it, then they should continue with it. That’s the logic. They are trying to convince us that we should give ‘Zolgensma’ to children as early as possible, but they are doing everything to prevent us from getting it. According to Sergey Kutsev, director of the Academician NP Bochkov Medical Genetic Research Center and chief external specialist of the Ministry of Health of the Russian Federation in medical genetics, many parents of children with “Zolgensma” currently have “somewhat inflated” expectations. Some perceive the drug as a “magic wand,” heavily influenced by the fact that it is administered only once. But it’s not that simple, says an expert from the Ministry of Health.

The medical commission always evaluates the child’s condition. It’s a delicate matter,” says Kutsyev. “There are various concomitant diseases or conditions that can have serious consequences after taking the drug. And the most obvious thing: if the patient is already undergoing pathogenetic therapy with other drugs and they are effective, there is no need to change it. It is important to consider the stage of disease progression. A geneticist gives an example: in the early stages of SMA, a child hypothetically retains 600 million motor neurons. According to the experimental data presented by Kucev, “Zolgensma” “saves” about half of them. And that is enough for the child’s development. “But in later stages, only 60 million neurons may remain alive. Even if Zolgensma preserves half of them, that is very little,” he explains.
This is why, in his opinion, “under certain circumstances, a prolonged rather than a single administration of drugs may have a better effect”. Kutsev points out that after the criteria for prescribing the drug “All Around Goodness” were expanded, a “great responsibility” was placed on the shoulders of doctors.
“Doctors are human too – they think, ‘God forbid something happens. They cannot experiment. There was a case in Russia where a child almost died after the introduction of “Zolgensma”. And it often happens here that parents make the decision to prescribe the drug. This is wrong,” said the head of the medical-genetic center.
The same opinion is shared by Oksana Ducevich, a counselor at the hotline of the “Families SMA” fund. She told the Russian service of the BBC: “Doctors consider what is best for the child. It is not necessary to think that if you have money, you can give the child any medicine”. The “Circle of Good” fund and Novartis could not be reached for comment in time.
On February 4, the charitable fund clarified its position on its official website – according to representatives of the organization, it will always be guided by the position of doctors. In the press service of the Kuban Ministry of Health it was emphasized that the decision to refuse the appointment of “Zolgensma” was made by the “Federal Committee”. Specialists of the Russian National Research Medical University named after N.I. Pirogov, the Russian Children’s Clinical Hospital and the National Medical Research Center for Children’s Health took part in it.
Malhaz Ugrehelidze is confident that there are no contraindications for administering “Zolgensma” to his son: “We meet all the criteria – the child weighs 15 kg, there are two copies of the SMN2 gene in the body, and there are no antibodies to adenovirus.” But if the medical commission gives the family a “reasoned answer” against taking the drug, then the boy’s father is ready to offer a “public apology”.
“I am not an enemy of my own son,” he says. “I asked the doctors: please give an official conclusion that the medicine is not suitable for the child. Explain why you think so. I will check it and possibly agree. If there is a justified position, I will publicly apologize and admit that I was wrong. But give us concrete criteria! And what do you write to us? ‘Not recommended,’ that’s it. Is that a medical criterion?” According to the father, Ugrehelidze plans to file a complaint against the doctors’ actions with the Investigative Committee in the near future. The prosecutor’s office has already opened an investigation at their request. The parents are ready to sue the doctors in the future.
The option of going abroad for medical treatment is not considered by the family. This would require new – and considerable – expenses. “The price of Zolgensma in Russia is fixed and amounts to about 121 million rubles,” Malkhaz Ugrekhelidze calculated. “We have collected it. Abroad, the drug is more expensive: 2.2 million dollars, or 167 million rubles. Do we have to ask people for money again? Why? Because they cannot prescribe the medicine here for our own money?”

